Appendix A: Microwave Heating of Lunar Materials
Introduction
Microwave heating of nonmetallic inorganic material has been of interest for many years. Von Hippel in the late 1940s and early 1950s investigated how microwave radiation up to 10 GHz couples to various insulator materials. Perhaps the most work has been done by Wayne Tinga at the University of Edmonton (Alberta, Canada). Most of the work to date has been done at the two frequency bands allowed in industrial use (0.915 GHz and 2.45 GHz). However, some work has recently been carried out at 28 GHz* and 60 GHz (Meek et at. 1986). At Los Alamos National Laboratory, the work has centered about the fabrication of useful engineering components.
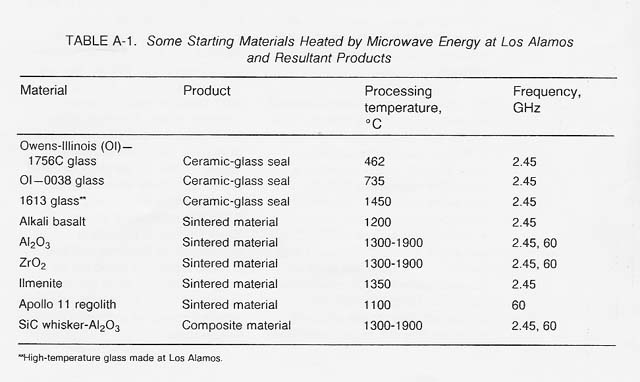
Table A-1 lists some materials that have been thermally processed using microwave energy and some products that have been fabricated at both 2.45 GHz and 60 GHz.
*Personal communication with H. D. Kimrey, Fusion Energy Division, Oak Ridge National Laboratory.

Using microwave energy to process lunar material offers a new, potentially very efficient way of heating these types of materials. Not only can lunar material be heated with less energy than that required by conventional methods, but the heating is accomplished more uniformly and in much less time (Meek et al. 1985).
Discussion
Many oxide materials are transparent to microwave energy at 2.45 GHz and 0.915 GHz. Oxides that possess impurities, such as mobile ions or mobile defects, that enhance their electrical conductivity will, however, couple to electromagnetic radiation in this frequency range. Heating will be primarily electronic.
For example, beta alumina contains by weight 11 percent sodium, thus enabling it. to couple efficiently to 2.45 GHz microwave radiation. Beta alumina, when placed in a 2.45 GHz microwave field of 400 watts power can be heated from room temperature to its sintering temperature (1850°C) in just a few seconds (Berteand and Badot 1976). Materials such as cuprous oxide (CU20), zinc oxide (ZnO), and zirconium dioxide (ZrO2) will also couple efficiently because they are defect-controlled semiconductors. To heat traditional oxide materials, such as alpha alumina, we incorporate materials that do couple to 2.45 GHz radiation, such as aluminum nitrate. These materials cause the oxide to heat to a few 100 degrees Celsius, after which the oxide will couple because its ability to absorb electromagnetic energy (its loss tangent) has increased sufficiently.
It is known that most lunar regolith, down to a depth of 3 meters, contains at least 106 imperfections per cubic centimeter from cosmic rays, solar flares, and the solar wind (see fig. A-1). The defects introduced into this soil over millions of years' exposure 10 these high-energy particles should increase the loss tangent of this material and allow it to be heated in a microwave field without the use of coupling agents. Terrestrial alkali basalt shows only weak coupling initially; however, when the intensity of the electric field is increased, this material heats rapidly. Recently we demonstrated the ability to heat ilmenite to its melting temperature using 2.45 GHz microwave energy. Since ilmenite is present in: abundance on the lunarsurface in mare regions, it could act as a coupling agent to allow 1he initial heating of those lunar materials that may not couple at ambient temperature.
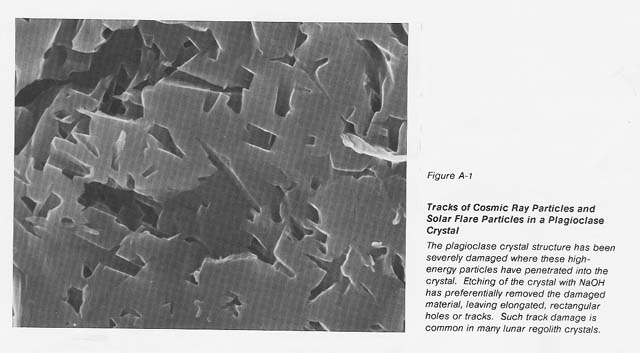
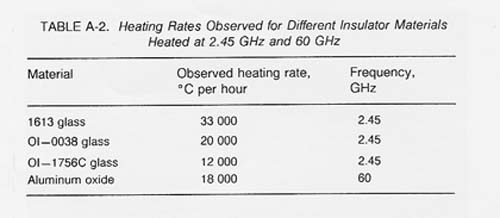
Table A-2 shows observed heating rates for some of the materials thermally processed using 2.45 GHz and 60 GHz microwave energy. If the proper electric field intensity (E) or magnetic field intensity (H) is used, rapid heating of lunar materials will also occur. The following expression (Puschner 1966) shows the relationship between the approximate heating rate and the applied electric field intensity for the heating of an insulator material.
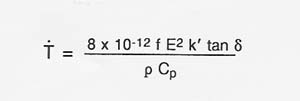

Because heating on the Moon will occur in a vacuum, where much greater electric field intensities can be used, materials that would not couple on Earth may be heated very easily and quickly.
Recommendations
Much work remains to fully characterize some of the phenomena observed to date with microwave-heated oxide and composite materials. For example, diffusion should be modeled, reaction kinetics should be studied, and sintering kinetics should be better understood.
References
Berteand, A. J., and J. C. Badot. 1976. High-Temperature Microwave Heating in Refractory Materials. J. Microwave Power 11 :315-320.
Meek, T. T., et al. 1986. Microwave Processing of Ceramics. J. Microwave Power & Electromagnetic Energy 21: 193-194.
Meek, Thomas T,; David T. Vaniman; Franklin H. Cocks; and Robin A. Wright. 1985. Microwave Processing of Lunar Materials: Potential Applications, In Lunar Bases and Space Activities of the 21st Century, ed,.W. W. Mendell, 479-486. Houston: Lunar & Planetary Inst..
Puschner, Herbert. 1966. Heating with Microwaves: Fundamentals, Components, and Circuit Technique. Philips Technical Library. New York Springer-Verlag.
Tinga, W. R. 1970. Interactions of Microwaves with Materials. Proc. IMPI Short Course for Users of Microwave Power, pp. 19-29, Nov.
Tinga, W. R., and E. M. Edwards. 1968. Dielectric Measurement Using Swept Frequency Techniques. J. Microwave Power 3:144-175.
Tinga, W. R., and S. O. Nelson. 1973. Dielectric Properties of Materials for Microwave Processing- Tabulated. J. Microwave Power 8:23-66.
Tinga, W. R., and W. A. G. Voss. 1968.. Microwave Power Engineering, vol. 2, pp. 189-194. New York: Academic Press.
Von Hippel, Arthur R., ed. 1954. Dielectric Materials and Applications. Cambridge, MA: M.I.T. Press.
Von Hippel, Arthur Robert. 1954. Dielectrics and Waves. New York: John Wiley & Sons.
![[NASA]](images/NASAball.gif) ![[Ames Research Center]](images/arclogo1.gif) |
WebWork: Al Globus, Bryan Yager, and Tugrul Sezen |
![[LifeSciences]](images/lslogot.gif) ![[Space Settlement]](images/splogosm.gif) |