
Heap leaching: This operation is identical in principle to the dump process but employs crushed, medium-grade ores piled on an impermeable basin from which the leachate is recovered, the solute is removed from it, and the leachate is concentrated and recycled (see fig. 31). This leaching operation is usually completed in a matter of months. To encourage the desired aerobic state, aeration tunnels are generally installed.

Vat leaching: Vat leaching is a purely chemical process by which concentrates of copper oxide ores are extracted by agitation with measured volumes of sulfuric acrd. Research indicates that sulfidic ores can be extracted economically by the vat process using bacterial leaching (Brierley 1982, Torma and Banhegyi 1984).
In situ leaching: In selected mining sites that are inaccessible or abandoned because the high-grade ore has been recovered, acid leaching solutions are applied directly to either shallow or deep deposits and the leachates subsequently collected via wells. The process is confined to copper and uranium ores. Naturally occurring bacteria may augment this chemical process. It has been speculated that populations of appropriate thiobacilli and other sulfur-oxidizing bacteria could be injected into the locus to hasten and enhance the -extraction. However, such a method is beset with difficulties, largely because the pathway of percolation is impossible to predict or control, thus endangering the quality of ground and surface waters. On the waterless Moon, the pollution of ground waters would not be a problem, but the extravagant use of water transported from Earth would be prohibitive.
In my opinion the only beneficiation operation having any potential for application on the Moon, or other space body, would be microbe- enhanced vat leaching, inasmuch as the bacteria must be provided with a confined, minimally sized Earth-like environment, as I have indicated. The oxygen for the bioprocessing unit would come from the reductive chemical processing of ilmenite, or other oxide ore, via electrolyzed water resulting from the reaction. Thus, chemical processing of lunar ores (or some other local means of producing oxygen) must precede any biobeneficiation for it to be economical. It seems unlikely that bioprocessing of ores would ever become a part of a closed or semi- closed regenerative ecological system unless the chemical beneficiation process proved to be ineffective (an unlikely prospect) and oxygen from eucaryotic photosynthesis could be spared for the bacterial processing of ores. Most likely, if cost-benefit analysis indicated any virtue to bioprocessing, the operation would be conducted outside of any human settlement and tended either by robots or humans in suitable "space suits."
Other Microbial Transformations of Metals
Anaerobic (Reducing) Conditions
In the absence of molecular oxygen, anaerobic or facultatively anaerobic bacteria are able to reduce sulfur or metals leading to the formation of sulfidic ores and reduced forms of iron, copper, and other metals.Sulfate-reducing bacteria: Two large groups of chemosynthetic, heterotrophic bacteria are able to oxidize certain organic acids and utilize sulfate, sulfur in other oxidized states, and elemental sulfur as electron acceptors. These bacteria, classified in such genera as Desulfovibrio, Desulfotomaculum, and Desulfuromonas, are found in anaerobic aquatic habitats containing the various oxidized states of sulfur, which are reduced to hydrogen sulfide, thus leading to the deposition of sulfidic ores.
Photosynthetic bacteria: Among the photosynthetic bacteria are two groups that utilize hydrogen sulfide under anaerobic conditions as a source of electrons in reducing carbon dioxide:
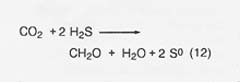
In one group, the "purple sulfur bacteria," the sulfur is deposited
intracellularly. In another group, the "green sulfur bacteria," the
sulfur is deposited outside the bacterial cell and subsequently oxidized to
sulfate in the presence of hydrogen gas (source of electrons). These, and other
photosynthetic bacteria, are anaerobic and thus unable to utilize oxygen as
the final electron acceptor. The purple sulfur bacteria comprise ten genera
(e.g., Thiospirillum, Thiocystis, and Amoebobacter); the green
sulfur bacteria comprise four genera
(e.g., Chlorobium and Pelodictyon).
Bacterial reduction of metal ions: Some strains of members of the genera Thiobacillus and Sulfolobus are able to reduce Fe+++ to Fe++, obtaining reducing power from elemental or reduced sulfur, which is oxidized in the process. A number of other soil bacteria are able to reduce ferric iron as well. Manganese is both reduced and oxidized by various marine and soil bacteria. Ferromanganese nodules found in the oceans are laid down slowly through the bacterial reduction and oxidation of ferric and manganese ions (Summers and Silver 1978, Ehrlich 1981, Ehrlich and Holmes 1986).
Biotransformations of Some Toxic Metals and Metalloids
Within any land or aquatic ecosystem, toxic and nontoxic cations, whether naturally or artificially introduced, may be absorbed and metabolized by certain species among the indigenous microflora. In the process, inherent toxicitins are often neutralized or modified. An excellent case in point is mercury which is highly toxic (binds sulfhydryl groups) as Hg + + but is enzymatically reduced by various mercury-resistant microorganisms to its volatile and nontoxic state as HgO (Belliveau et al.. 1987. Decker 1986. Hutchins et al. 1986. Thompson 1986).
Likewise certain toxic metals are methylated through the action of methylcobalamin excreted by a number of different aquatic and soil bacteria. This methylation does not necessarily detoxify the metal. In the case of mercury, the methyl and dimethyl forms can subsequently be concentrated in certain organisms, especially fish.. Other metals that can be methylated by bacteria include tin, cadmium, lead, and arsenic.
Some bacteria can cleave certain organic mercurials into Hg + + plus an organic residue. Arsenic as As3 + can be oxidized by a number of bacteria to As5 +; in either state of oxidation, arsenic is toxic, but far less so in the pentavalent state. Reduced arsenic reacts with sulfhydryl groups; oxidized arsenic simulates phosphate in metabolic pathways. As oxoanions, tellurium and selenium are toxic to many bacteria. Some bacteria are able to reduce these salts to Te0 or Se0, thereby destroying their toxicity.
Since lunar rock contains virtually the same spectrum of toxic metals as found on the Earth (Morris et al. 1983), the biobeneficiation of ores on the Moon could be compromised by the presence of toxic cations in ore slurries, unless the bacteria introduced into the vats are resistant to high levels of cations of the more abundant toxic metals. including those to be recovered. Resistance to toxic metals is genetically endowed and can be introduced through gene recombination techniques in at least some of the bacterial species involved in the beneficiation of ores or in the removal of toxic cations from an aquatic envirpnment (Belliveau et al. 1987. Ehrlich and Holmes 1986. Hughes and Poole 1989. Hutchins et al. 1986, Nicolaidis 1987. Pooley 1982, Torma 1987a, Trevors 1987. Tsezos 1985, Woods and Rawlings 1985).
Leaching of Ores by Growth of Heterotrophic Microorganisms
Sometimes sufficient organic matter is found in ores to support the growth of diverse microbes, resulting in the production of organic acids (e..g., lactic, citric, acetic, glutamic, and glycolic) which lower the pH, helping to solubilize the metals and encouraging the development of an acidophilic microflora of thiobacilli. This may be viewed as a synergistic effect.
On the other hand, alkaline leaching may be encouraged in the presence of organic nitrogen (protein, amino acids, purines, pyrimidines, etc.) as a result of deaminations catalyzed by heterotrophs, yielding ammonia which, in aqueous solution, becomes ammonium hydroxide. Some metals, notably copper, cobalt, and zinc, are compounded by ammonium hydroxide.
Calculations suggest it is not feasible to harness heterotrophs for the leaching of ores. Enormous quantities of decomposable organic matter would need to be provided in order for either acid or alkaline leaching to function at a commercial level (Kelly, Norris, and Brierley 1979). The use of organic wastes from sewage treatment plants has been investigated (Hutchins et al. 1986).
Bioaccumulation of Metals
Some microorganisms are capable of assimilating large amounts of metals from solution. The outlook for exploitation of such organisms for the removal of toxic ions or for the concentration of useful metals is very bright indeed (Ehrlich and Holmes 1986, Hughes and Poole 1989, Torma 1987a and b, Volesky 1987). Of course, all living creatures require certain trace elements which are found in low concentrations within all cells, though they would be toxic in higher concentrations. Certain microorganisms, however, are endowed with the capacity to assimilate large amounts of certain metals, even toxic ones.
Electrostatic Attraction
A variety of functional groups, or ligands, on cell surfaces carry positive or negative charges, conveying a net charge to the cell. Other things being equal, the intensity and sign (+ or -) of the charge depends on the pH of the extracellular environment. In most natural environments the pH is higher than the cell's isoelectric point; therefore, the cell will have a net negative charge and will passively attract cations, much like an ion exchange resin. However, there is some selectivity, suggesting the existence of specific binding sites for particular cations on the various surface structures. Certain fungi and bacteria, for example, Bind large quantities of uranium ions, in some instances to the extent of 15 percent of the cells' dry weight. A yeast was found to bind mercury to its cell walls in amounts equivalent to the weight of the cell walls themselves. Certain species of algae and fungi concentrate copper to the extent of 12 percent of the cells' dry weight. Other metals bound by electrostatic attraction include Fe, La, Cd, Ca, Mg, Pb, Ni, Mn, Zn, Ag, K, and Na.
Surface Deposition or Precipitation (Biosorption)
Massive amounts of metals or insoluble metal compounds can be deposited on the surfaces of some microorganisms. This deposition will occur in some instances when the metal is metabolized; in other instances no transformation of the metal is required for its deposition (Belliveau et al. 1987, Ehrlich and Holmes 1986, OlsCi1n~nd Kelly 1986, Thom~son 1986),.
Those species of bacteria that reduce tellurite or selenite to metallic Te or Se deposit the metals on their surfaces, accumulate them intracellularly, or both. Some bacteria will aggregate insoluble lead compounds on their surfaces. Most common is the precipitation of ferric compounds and manganic oxides. While many bacteria can oxidize the manganous ion, sheathed, filamentous bacteria in the genera Hyphomicrobium and Metallogenium and in the Sphaerotilus-Leptothrix group become heavily coated with manganic oxides. Also, Sphaerotilus-Leptothrix and a group of stalked bacteria in the genus Gallionella acquire heavy deposits of oxidized iron, largely ferric hydroxide. Apparently, gallionellae derive energy through the oxidation of Fe + +, while the sheathed, filamentous group merely attract the insoluble ferric hydroxide to their sheaths. The genus Zoogloea, which is commom'i, in activated sludge sewage operation systems, produces copious quantities of polysaccharide slime having high affinity for Cu+ +, Cd+ +, Co+ +, Ni+ +, Zn+ +, and Fe+ + + (Hutchins et al. 1986). The production of extracellular polysaccharide slimes is common to many bacteria; chemically, they vary considerably from species to species, even from strain to strain (Ehrlich and Holmes 1986, Thompson 1986, Torma 1987a, Volesky 1987).
Intracellular Transport of Ions
Both monovalent (Na +, K+, Gs +, Li+, Tl+, and NH4+) and divalent cations are specifically transported to satisfy nutritional needs of microorganisms. In some cases, rather high intrqcellular concentrations of certain metals are achieved. Often, the same transport mechanism will function for several cations; e.g., Mg ++, Co++, Mn+ +, Ni+ +, and Zn+ + in Escherichia coli. Others are more specific, although in all such systems various other cations will compete with a particular cation for uptake, in some cases even inducing efflux of ions. Fungi appear to concentrate metals or metalloids to a somewhat greater extent than do most bacteria (Hutchins eta.!., 1986, Summers and Silver 1978, Tsezos 1985).
Many of the metal ions are toxic, although the toxicity varies considerably from species to species (Belliveau et al. 1987, Ehrlich 1981, Summers and Silver 1978). Sometimes the inhibition of bacterial growth is synergistic. For example, Klebsiella aerogenes has been shown to be inhibited to a greater extent by Cd and Zn than by the sum of the individual toxicities of the two cations. The presence of clays, certain anions, and organic matter of various kinds often markedly reduces metal toxicity. Not surprisingly, metal chelators (e.g., ethylenediamine tetraacetic acid and citric acid) suppress the toxicity of many cations toward microorganisms. Clearly, toxicity is an important consideration in harnessing microbial cells to concentrate metals or metalloids.
An interesting case is found with the diatoms, which encase themselves in siliceous shells in an amazing variety of beautiful shapes. The uptake of silicates by diatoms has been shown to be competitively inhibited by germanic acid, thereby suggesting a means of recovering germanium from natural sources (Kelly, Norris, and Brierley 1979; Krumbein 1983). (See below for another aspect of silicate uptake.) Incidentally, the Russians for over 20 years have referred to "silicate bacteria," which they claim, in English abstracts, will free silicates from aluminosilicate ores, thereby beneficiating aluminum oxide (Alexandrov, Ternovskaya, and Blagodyr 1967; Andreev, Lycheva, and Segodina 1979; Andreev, Pol'kin, and Lyubarskaya 1982; Rohatgi, Trivedi, and Rohatgi 1984).
Not to be overlooked is the uptake of oxoanions, especially sulfate, the transport of which has been shown in Salmonella typhimurium to be competitively inhibited by chromate, selenite, molybdate, tungstate, and vanodate (descending order of effectiveness) .
Natural Chelators
Some naturally occurring, low molecular weight compounds (citric acid, aspartic acid, and a number of dicarboxylic acids) have long been known to chelate various metal ions. Many microorganisms produce organic compounds that can do the same thing. Such organic compounds are collectively called "ionophores," the best known of which are siderophores (iron-attractors) (Nielands 1981; Brock, Smith, and Madigan 1984). Microbes that produce siderophores are believed to have evolved as the rising oxygen content of the Earth's atmosphere caused oxidation of much of the iron into its insoluble oxide and hydroxide states. To capture the minute quantities of free ferric ions that exist, microbes capable of synthesizing chelators of ferric iron arose. Incidentally, animals (including humans) trap iron through iron-binding proteins such as lactoferrin, transferrin, and ferridoxin, the latter being particularly abundant in the liver. Many pathogenic bacteria and fungi compete with the infected host for iron through the formation of siderophores. The siderophores- phenolates, hydroxamates, or cyclic peptides-are capable of binding ferric iron, which is subsequently transported into the cell, released, reduced enzymatically, and secreted back into the environment for recycling.
Recently, an ionophore for silicate, another ion very sparingly available, has been found in diatoms (Bhattacharyya and Volcani 1983).
Other metal-binding organic molecules, not chelators, abound in living systems. Of particular note is metallothionein, which binds a variety of cations by virtue of its available sulfhydryl (-SH) groups (Kagi and Kojima 1987).
![[NASA]](images/NASAball.gif)
![[Ames Research Center]](images/arclogo1.gif)
|
WebWork: Al Globus, Bryan Yager, and Tugrul Sezen |
![[LifeSciences]](images/lslogot.gif)
![[Space Settlement]](images/splogosm.gif)
|