Lunar Cement
William N. Agosto
With the exception of water, the major constituents of terrestrial cements are present at all nine lunar sites from which samples have been returned.
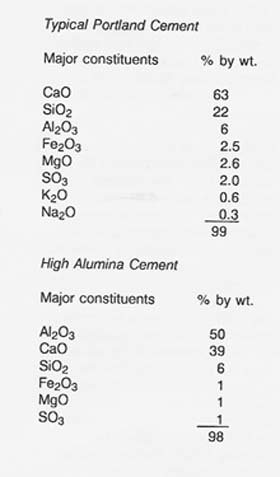
Two examples of the most commonly used cement formulations (Mindess and Young 1981) are listed below.

All the above oxide constituents are found in lunar soils, basalts, and anorthosites except that iron oxide is in the ferrous form (FeO) and sulfur occurs as FeS in basalts and in sparsely scattered particles of meteoritic metal in the soil. However, with the exception of relatively rare cristobalite (SiO2), these oxides are not present as individual phases but are combined in silicates and in mixed oxides [e.g., ilmenite (FeTiO3)].
Lime (CaO) is most abundant on the Moon in the plagioclase (CaAl2Si203) of highland anorthosites. The anorthosites are approximately 90 percent anorthite containing 20 percent lime by weight (McKay and Williams 1979).
It may be possible to enrich the lime content of anorthite to levels like those in Portland cement by pyrolyzing it with lunar-derived phosphate in a reaction like

If workable, reaction (1) increases lime content from 20 percent by weight in anorthite to 50 percent by weight in the slag. A similar reaction has been proposed by Ellis M. Gartner of Construction Technology Laboratories, the research group of the Portland Cement Association. That reaction includes carbon as a reductant and uses apatite as the lunar phosphate. However, reductants may not be required in the high vacuum of the lunar environment. Both phosphate reactions need to be thermodynamically tested at the bench level.
Phosphate consumed in reaction (1) can be regenerated by reacting the phosphorus product with lunar augite pyroxenes at elevated temperatures in a reaction like
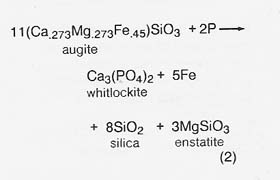
Colleagues and I (1980) have suggested that reaction (2) may be the process generating phosphate in certain stony-iron meteorites (the mesosiderites). In the meteorites, phosphorus is derived from meteoritic metal, where it occurs: as a phosphide in an intermetallic phase. The same phosphide phase is found in metallic particles of lunar soil, which are derived from meteorites. These can be magnetically beneficiated as a source of iron, nickel, phosphorus, and cobalt (Agosto 1981). The soil metal contains as much as 11.5 percent by weight phosphorus, with an average of about 1 percent phosphorus (Goldstein and Axon 1973). Lunar phosphate has also been found as a minor component of KREEP (potassium, rare earth elements, phosphorus) soils and basalts, notably Apollo 14, at a maximum of about 1 percent by weight.
Thus, the lunar soil could provide both the phosphate needed for reaction (1) and the reduced phosphorus needed for reaction (2). Alternatively, terrestrial phosphate could be transported to space as a reagent, with phosphorus losses in recycling replenished from lunar sources.
The oxide components for Portland cement may also be produced from the silicate minerals in the abundant lunar mare pyroxenes [like the augite used in reaction (2)] by the vacuum solar pyrolysis used in reaction (1) but without the need for a phosphate reagent (Agosto and King 1983). Sufficient sulfur for the formulation of cement might also be derived from the FeS in soil metal.
It may also be possible to obtain high alumina lunar cement directly by solar pyrolysis of anorthite derived from highland anorthosites. The oxide components of anorthite are alumina (Al203), lime (CaO), and silica (SiO2). In a solar furnace, the silica would vaporize preferentially, leaving a residue enriched in lime and alumina within the composition range of high alumina cements (Agosto and Gadalla 1985).
References
Agosto, W. N., and E. A. King. 1983. In Situ Solar Furnace Production of Oxygen, Metals and Ceramics From Lunar Materials. In Lunar & Planetary Sci. XIV, Special Session Abstracts: Return to the Moon (March 16) and Future Lunar Program (March 17), pp. 1-2. Houston: Lunar & ptanetary Inst.
Agosto, William N. 1981. Beneficiation and Powder Metallurgical Processing of Lunar Soil Metal. In Space Manufacturing 4, Proc. 5th Princeton/AIM Conf., ed. Jerry Grey and Lawrence A. Hamdan, 365-370. New York: AIM.
Agosto, William N., and Ahmed M. M. Gadalla. 1985. Lunar Cement Form~lations for Space Systems Shielding and Construction. In Space Manufacturing 5: Engineering with Lunar and Asteroidal Materials, Proc. 7th Princeton/AIM/SSI [Space Studies Institute] Conf., ed. Barbara Faughnan and Gregg Maryniak, 179-183. New York: AIM.
Agosto, William N.; Roger H. Hewins; and Roy J. Clarke, Jr. 1980. Allan Hills A77219, the First Antarctic Mesosiderite. Proc. 11th Lunar & Planetary Sci. Cant., 1027-1045.
Goldstein, J. I., and H. J. Axon. 1973. Composition, Structure, and Thermal History ot Metallic Particles From 3 Apollo 16 Soils, 65701, 68501, and 63501. Proc. 4th Lunar Sci. Cont. Suppl. 4, Geochim. Cosmochim. Acta, 751-775.
McKay, David S., and Richard J.. Williams. 1979. A Geologic Assessment of Potential Lunar Ores. In Space Resources and Space Settlements, ed. John Billingham, William Gilbreath, and Brian O'Leary, 243-255. NASA SP-428.
Mindess, Sidney, and Francis J, Young. 1981. Concrete. Englewood Cliffs, NJ: Prentice- Hall, 671 DD.
![[NASA]](images/NASAball.gif) ![[Ames Research Center]](images/arclogo1.gif) |
WebWork: Al Globus, Bryan Yager, and Tugrul Sezen |
![[LifeSciences]](images/lslogot.gif) ![[Space Settlement]](images/splogosm.gif) |