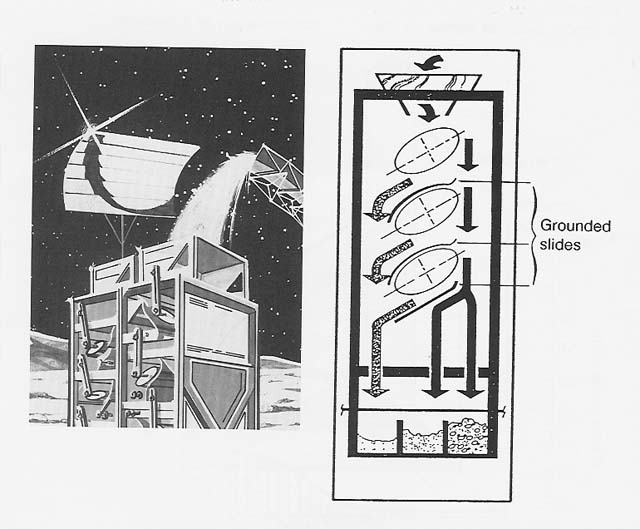
Agglutinates are the major component of lunar soil,"making up, on the average, 50 percent of the regolith. Most of the agglutinates contain finely dispersed metallic iron, which gives them a broad magnetic range that overlaps the magnetic susceptibility of other soil components, including ilmenite. For that reason, it is difficult to separate agglutinates from ilmenite by magnetic means. However, the nonconducting behavior of the agglutinates allows them to be separated from ilmenite electrostatically. During separation, the soil sample was heated to approximately 150°C to drive off terrestrial water and enhance the contrast in conductivity between ilmenite and other mineral components (see fig. 3).

Figure 3
A Commercial Electrostatic Separator on the Lunar Surface
The mirror in this concept of an electrostatic separator on the Moon focuses solar radiation on the soil to be separated. Heating to about 100°C will increase the conductivity of the semiconductor ilmenite while leaving the conductivity of insulators like agglutinates unchanged. The enhanced contrast in conductivity increases the separability of these components of the lunar soil.
In the schematic. the arrows indicate the path of the soil feed and how it separates. The ellipses are cross sections of the high-voltage electrodes. The nonconducting materials fall to the far right. The middlings fall in the middle. The conducting and semiconducting materials fall to the far left.
In my electrostatic studies the lunar anorthite seemed to collect preferentially close to the grounded electrode (the feed hopper), but the slide design of the separator was not configured to take advantage of that.
The slide did enhance the density segregation of ilmenite by air (nitrogen) resistance. Ilmenite is almost twice as dense as the other major soil components. Accordingly, electrostatic separation of the ilmenite in a vacuum, where air resistance was not a factor, was not as successful, and the mineral reached a maximum concentration of only 30 percent in one pass under those conditions (fig. 4). This, however, amounts to a fourfold increase compared to the starting concentration of 7 percent.
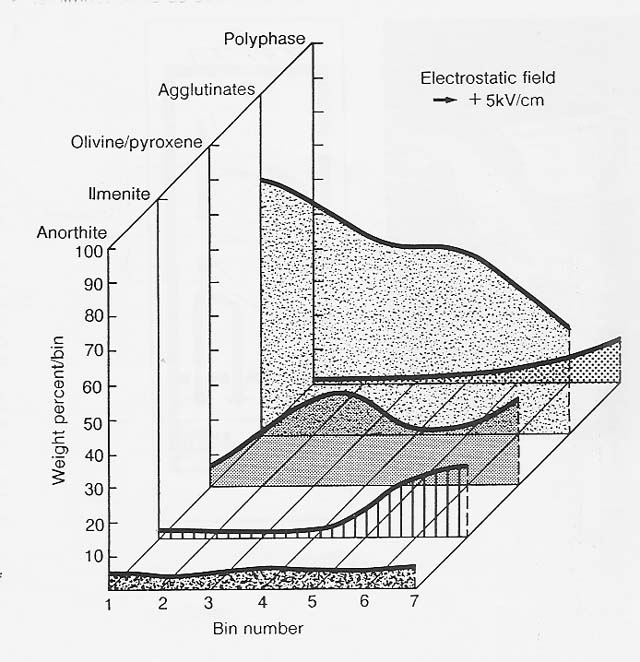
Figure 4
Electrostatic Separation in a Vacuum of Apollo 11 Soil 10084,853, 90.150 pm Fraction
To improve yield in the vacuum of the lunar environment would require redesign of the electrostatic separation apparatus. A vertical, free-fall design (as seen in fig. 3) might be the best approach for separating lunar soil minerals according to their electrical behavior and would be especially appropriate in the lunar environment, where fall times are about twice what they are on Earth.
![[NASA]](images/NASAball.gif)
![[Ames Research Center]](images/arclogo1.gif)
|
WebWork: Al Globus, Bryan Yager, and Tugrul Sezen |
![[LifeSciences]](images/lslogot.gif)
![[Space Settlement]](images/splogosm.gif)
|