Oxygen From the Lunar Soil by Molten Silicate Electrolysis
Russell O. Colson and Larry A. Haskin*
Introduction
In 1835, a lunar fantasy published as a factual account in the New York Sun (see French 1977) generated great economic interest in the Moon. This fantasy reported, among other things, the existence of huge gems on the Moon. Within a few weeks the story was shown to be a hoax, but the interest it generated remained. Now, the Apollo missions have dimmed hopes that such traditional treasures will be found on the Moon. These missions provided no evidence that the Moon ever experienced the plate tectonic processes or the major water and gas transport processes which have produced most gem minerals and ore deposits on Earth. Even if gem minerals and ore deposits had formed, they would probably have been destroyed or dispersed by meteoroids hitting the surface of the Moon. Besides, the cost of acquiring gems from the Moon will be prohibitive for the foreseeable future; if the most common lunar rocks were sold as souvenirs, they would carry a higher price than rubies.
Nevertheless, just as it was not the fantastic Fountain of Youth but rather the real land and plentiful natural resources that proved to be the wealth of the New World, so the treasures of the Moon will be found in less romantic notions. "Water is worth more than gold to a parched desert wanderer," runs a trite statement. But the statement is true for the Moon, and for the Moon can be extended to include oxygen. In fact, production of lunar oxygen for life support and fuel in low Earth orbit and beyond is already seen as an economic incentive to build a Moon base (e.g., Mendell 1985).
In general, because of the high energy cost of launching material into space from Earth's substantial gravity well, materials already in space (on the Moon, for example) gain value for construction projects there. Such materials could be used" as found" or after only simple processing. Lava tubes on the Moon could serve as early lunar shelters (Harz 1985). In near-Earth space, lunar basalt could be used as heat shielding for vehicles reentering the Earth's atmosphere and could provide built-in protection for orbiting platforms (e.g., Nozette 1983). More extensively processed bulk materials for space construction might include concrete (see Lin 1985 and also Lin's subsequent paper in this volume) and glass (Blacic 1985) from the Moon.
Also because of the high cost of exporting materials from the Earth to the Moon, it is reasonable to imagine that a Moon base would, in the longer term, approach self- sufficiency, as eloquently proposed in the book Welcome to Moonbase (Bova 1987). What lunar resources make such a scenario feasible? The answer is found in the most common materials and conditions known on the Moon-the soil, the rocks, and the reliable supply of sunlight.
All the common soils on the Moon are rich in oxygen (about 45% by weight), silicon (about 21 %), iron (6 to 15%), aluminum (5 to 13%), calcium (8 to 10%), magnesium (about 5%), and titanium (up to 6%). No ore bodies like those found on Earth have been proven to exist on the Moon, but some rock types have concentrated certain minerals; namely, ilmenite (rich in iron, titanium, and oxygen), anorthite (rich 1n calcium, aluminum, silicon, and oxygen), and olivine and pyroxene (rich in magnesium, iron, silicon, and oxygen). The chemical elements these soils and minerals contain are valuable in applications that range from construction to propulsion. And it may be economically possible to extract volatile elements present at low concentrations (such as carbon, hydrogen, and nitrogen) by heating the lunar soil (e.g., Haskin 1990).
The most promising early use for lunar resources is likely to be for energy. Energy for space transportation can come from lunar oxygen and hydrogen. Proposed energy exports include solar energy collected on the Moon and converted to microwaves (e.g., Criswell and Waldron 1985) and fuel in the form of 3He for nuclear energy (e.g., Wittenberg, Santarius, and Kulcinski 1986) as well as chemical propellants (e.g., Thompson 1951, Arnold 1980, Mendell 1985). Considerable interest in chemical propellants has revolved around the extraction of oxygen (Mendell 1985), the most abundant and possibly the most immediately valuable of these lunar energy resources. It will be used as oxidant for fuel in the Earth- Moon system, and perhaps ultimately for flights to Mars if it can be provided at less cost than oxygen brought from the Earth (see, for example, Davis 1983 and Mike Simon's paper in volume 2, "Utilization of Space ResQurces in the Space Transportation System").
Oxygen From the Moon
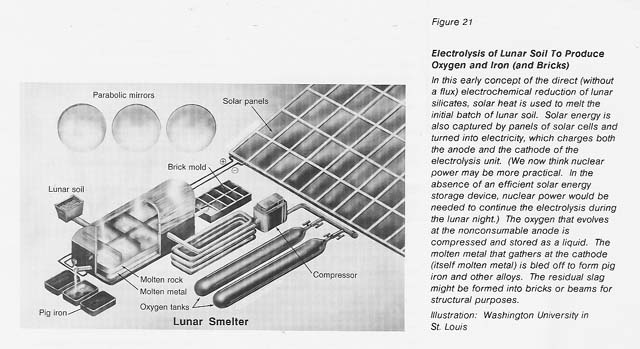
Accepting, then, that oxygen, rather than gigantic gems or gold, is likely to make the Moon's Klondike, we have chosen to investigate the extraction of oxygen from the lunar soil. Unlike the Klondike, this strike will not be made by the prospector who discovers the location of the oxygen ore, for it is everywhere on the Moon. This strike will be made by the inventor who discovers the robust, economical process for extracting the oxygen. We think the process that will pan out will be electrolysis of molten soil. (See figure 21.) We are investigating it because it is conceptually simple and because its nontraditional character fits the nontraditional lunar materials and conditions.

In our study of molten silicate electrolysis, we have taken the approach that the first step in developing the process is to understand its fundamental chemistry. This includes understanding the primary reactions that take place at the electrodes, the kinetics and energies of those reactions, competing reactions that might reduce the efficiency of oxygen production, and how melt resistivity changes with temperature and melt composition. The answers to these questions tell us whether the process is theoretically viable and competitive, presuming appropriate technologies can be developed to implement the process in real life and minimize dynamic problems arising during electrolysis (such as remixing of the product with the feedstock or insufficient mixing of the silicate melt). We have also begun investigating other specific questions about s.jlicate melt electrolysis, such as durability of container and electrode materials and the nature and composition of the product. Our results are summarized here and are reported in more detail in Haskin et al. 1990,
Colson and Haskin 1990, and
Haskin and Colson 1990
*Department of Earth and Planetary Sciences and McDonnell Center for the Space Sciences, Washington University, St. Louis, MO 63130. We thank the National Aeronautics and Space Administration for partial support of this work through the NASA/University of Arizona Space Engineering Research Center.
![[NASA]](images/NASAball.gif)
![[Ames Research Center]](images/arclogo1.gif)
|
WebWork: Al Globus, Bryan Yager, and Tugrul Sezen |
![[LifeSciences]](images/lslogot.gif)
![[Space Settlement]](images/splogosm.gif)
|