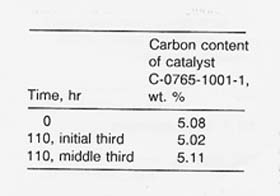
Catalyst Life
The catalyst was still active when it was removed after 14 runs (110 hr). As can be seen from the tabulation below, analyses of the catalyst before and after use showed no carbon deposition.

As stated previously, there was no pressure buildup during the run, so this would not be a limiting factor on the life of the catalyst. However, impurities in the feed may prove to be a limiting factor. Temperature control is also vital, because carbon is definitely deposited on the catalyst at higher temperatures (400°C and up). Catalyst life would probably be extended if the operating temperatures were started low when the catalyst was new and active and then gradually raised as the catalyst activity declined.
Catalyst Bed Length
At low space velocities, only the first inch or two of the catalyst bed was involved in the major portion of the reaction. As the space velocity was increased, more and more of the bed was involved until, at very high space velocities and low hydrogen-carbon monoxide mole ratios (runs 55 and 57), even the full length of the catalyst bed was unable to achieve complete conversion of carbon dioxide into methane and water. This effect is best shown by carbon dioxide gradients in the reactor taken for the various runs, as reported in table 3. Two additional advantages of a long catalyst bed are that it allows a margin of safety as the catalyst ages and becomes less active and that it allows the initial portion of the bed to act as a guard chamber to remove various catalyst poisons.
Lunar Surface Plant Design
Estimates of the weight and power requirements for a lunar surface plant using the Aerojet carbo thermal process are given in this section of the paper. In making these estimates, we assumed that no water is present in, or obtainable from, the lunar material. Large differences in weight result when different cooling methods are employed, because of the large amount of waste heat produced.
Heat Rejection
Two different methods of heat rejection were considered in this study:
The first method is based on standard refrigeration principles. It employs n-butane as the primary refrigerant, with water as the secondary refrigerant and the medium for transferring heat to a space radiator. Refrigeration is not used in the second method. Instead, we assume that a radiator is able to reject heat directly into 0 Kspace.
In the estimates for the different sections of the process, power requirements are given for these two different methods of cooling. In the following tables and figures, method 1 indicates the refrigerative technique and method 2 indicates the radiative technique. The details of the two methods are discussed later in this paper.
Reduction of Silicates With Methane
The estimates of heat and power requirements are based on the following changes:

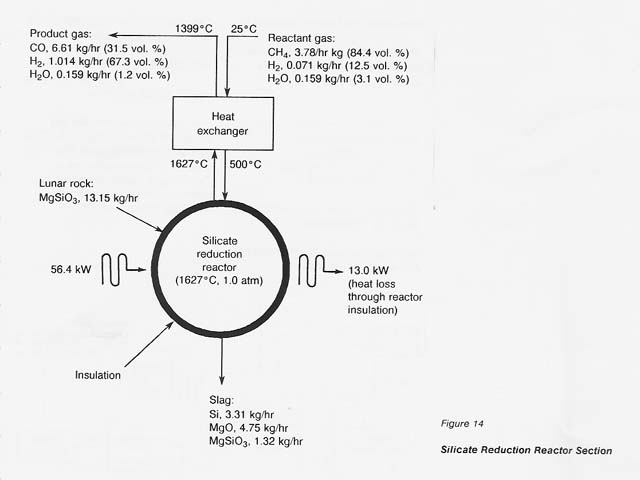
The process flowsheet for this section is shown in figure 14.
![[NASA]](images/NASAball.gif)
![[Ames Research Center]](images/arclogo1.gif)
|
WebWork: Al Globus, Bryan Yager, and Tugrul Sezen |
![[LifeSciences]](images/lslogot.gif)
![[Space Settlement]](images/splogosm.gif)
|