Processing of Metal and Oxygen From Lunar Deposits
Constance F. Acton
Metallurgical Processing
Introduction
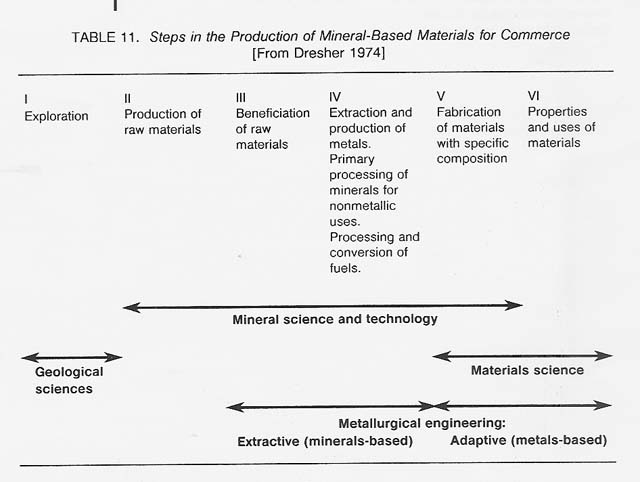
Metallurgy-the art and science of economically concentrating, extracting, refining, and fabricating metals and alloys - has existed on Earth from antiquity. Gold, silver, and copper-elements that can occur as metals in their natural state-were used as long as 10 000 years ago. Metals extraction technology can be traced back at least 6000 years. Recently, major advances have been made in metallurgical processing. This developed field may now be ready for application to the production of metals in space. Table 11 shows the steps involved in the production of mineral-based materials for commerce.

The first step is exploration to define potential sources of raw materials. The Apollo Program has defined some lunar resources; further exploration will undoubtedly find additional ones. Whether or not a metal-bearing deposit is classified as an ore, a reserve, or a resource is a question of economics. An ore is a resource that can be extracted at a profit. A reserve is a resource currently uneconomical to mine or greater than existing demand. A resource becomes a reserve or an ore if the proper technologies and economic conditions are developed.
The concentration of most metals in the crust of the Earth (or the Moon) is extremely low. And even the most abundant elements on Earth-iron, aluminum, silicon- cannot generally be extracted from common rock at a profit. A metal must be sufficiently concentrated in a mineral before it is mined. Then the useful constituents of an ore must be separated from the residue (gangue) by the process of beneficiation. In this process, the ore minerals are concentrated by physical separation, exploiting differences in such properties as particle size, shape, and size distribution; specific gravity; magnetism; and electrostatics.
The importance of the beneficiation step cannot be overemphasized. Only after the ore minerals have been concentrated can economical metal extraction take place. On the Moon, some whole rocks may be ores for abundant elements, such as oxygen, but beneficiation will be important if metallic elements are sought from raw lunar dirt.
In the extraction process, a beneficiated metallic ore, such as an oxide, sulfide, carbonate, or silicate mineral, is converted to the reduced metal. Such minerals are the stable forms of metals in the Earth's or the Moon's environment. In the case of the important iron ore mineral magnetite (Fe304), the free energy of formation at 0 degree C is more than 120 kcal/mole O2 according to the reaction
3/2 Fe + 02 = 1/2 Fe304
This explains the natural tendency of metallic iron to rust in air; that is, to convert to its thermodynamically stable oxide form. A very useful pictorial representation of the relative stability of metal oxides is an Ellingham diagram (fig. 33). These diagrams are also available for chlorides, fluorides, and sulfides.
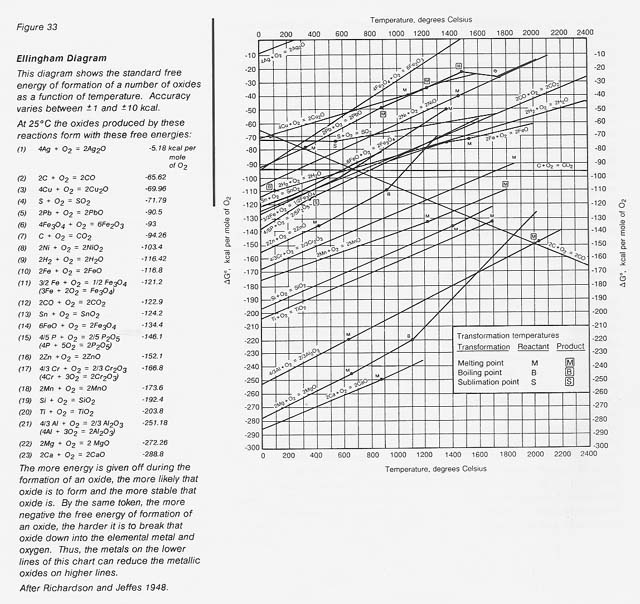
The larger the negative free energy of formation, the more stable the oxide. It can readily be seen that at 0 degree C the relative stability for oxides increases from iron through silicon and titanium to aluminum. The metals in the more stable oxides (lower on the chart) can chemically reduce the metals in the less stable oxides (higher on the chart). Also shown are lines for carbon and hydrogen, common reductants used in terrestrial extractive metallurgy. The challenge is to find procedures to extract the element of interest economically.
In some terrestrial cases, metals may be economically extracted at a higher than normal rate of energy consumption per unit of metal produced because cheap electrical energy is available; for instance, siting an aluminum smelter near a hydroelectric supply. In such a case, the rate of energy consumption may be 10-50 percent more than the general industry practice. The economics of utilizing metals from resources in space are driven by transportation costs rather than energy costs. Many processes proposed for extraction of lunar materials are energy-intensive. Thus, the cost of energy on the Moon will be an important factor in developing processing technology. For example, concentrated solar heat will be cheaper than electricity and should be utilized where possible.
Geochemical Availability
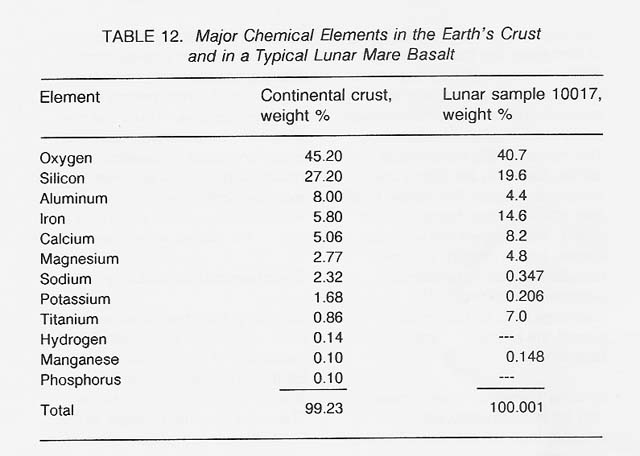
Skinner (1976) has provided a lucid analysis of the geochemical availability of various elements on Earth. The basic concepts are directly applicable to the Moon. The most abundant metals in the Earth's crust are silicon, aluminum, iron, calcium, magnesium, sodium, potassium, and titanium (see table 12). For comparison, the composition of a typical lunar mare basalt [sample 10017, as analyzed by Wanke et al. (1970)] is also shown. To a first approximation, the compositions of terrestrial and lunar rocks are not too different.
![[NASA]](images/NASAball.gif) ![[Ames Research Center]](images/arclogo1.gif) |
WebWork: Al Globus, Bryan Yager, and Tugrul Sezen |
![[LifeSciences]](images/lslogot.gif) ![[Space Settlement]](images/splogosm.gif) |