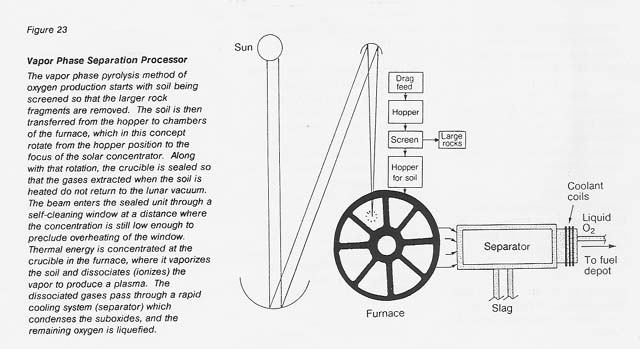
Vapor Phase Pyrolysis
Wolfgang Steurer
The vapor phase pyrolysis process is designed exclusively for the lunar production of oxygen. In this concept, granulated raw material (soil) that consists almost entirely of metal oxides is vaporized and the vapor is raised to a temperature where it dissociates into suboxides and free oxygen. Rapid cooling of the dissociate vapor to a discrete temperature causes condensation of the suboxides, while the oxygen remains essentially intact and can be collected downstream. The gas flow path and flow rate are maintained at an optimum level by control of the pressure differential between the vaporization region and the oxygen collection system with the aid of the environmental vacuum.
The process is illustrated in figure 23 in the form of a conceptual facility. The particle feedstock is dispensed from a gravity feed unit to a crucible. There it is vaporized and dissociated by means of thermal energy supplied by a solar concentrator whose focal point is at the crucible. The beam enters the vacuum-sealed unit through a self-cleaning window at a distance where the concentration is still low enough to preclude overheating of the window.

The dissociated gases pass then through a rapid cooling separator at a yet-to-be-determined optimum flow rate and flow pattern. The cooling column may, in reality, be considerably longer than shown in the sketch. The produced oxygen may be collected as gas in a balloon (with a shade/shield to protect it from the Sun's heat and from micrometeorite strikes) or liquefied. The problems of oxygen storage and liquefaction are common to all oxygen-producing processes and are, therefore, not addressed here.
The preferred raw material, in view of the ease of acquisition, is lunar basalt in the form of regolith (soil). Since it consists of a variety of metal oxides, numerous individual species are obtained during dissociation. For Si02, Ti02, and A1203. for example, the major (minor) species produced are
Si02 02 + SiO (+ Si02)
Ti02 02 + TiO (+ Ti02)
AlI203 02 + AlI02 (+ AIO + Al20 + Al202)
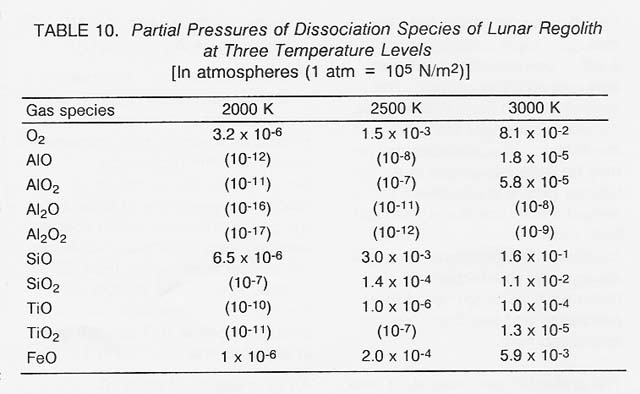
The relative importance of the individual dissociation species can be measured by their partial pressures which, in turn, increase rapidly with the processing temperature. For a mixture of oxides representative of lunar soil, the partial pressures of the species evolving from three major oxides in the temperature regime from 2000 to 3000 K are listed in table 10. (To convey a clear overview, negligible pressures below 1 0.6 atm are given in orders of magnitude only.) An examination of table 10 substantiates the following conclusions: (1) The processing temperature should be close to 3000 K (the limit for solar heating). (2) The gas composition is dominated by 02 and SiD. The oxygen pressure at 3000 K of roughly 1/10 of an atmosphere is more than adequate for production rates.
The oxygen yield as a fraction of the raw material throughput is determined from the partial pressure and molecular weight of each species by the relationship
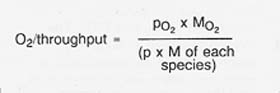
The resulting oxygen yield is on the order of 20 percent.
The processing energy required for vaporization and dissociation of the entire throughput is 5100 kWhr/t and for cooling 2000 kWhr/t, adding to a total of 7100 kWhr/t. Using the oxygen yield of 0.20, this translates into 35 500 kWhr/tonne of oxygen produced.
Most of this amount could be provided by solar thermal energy using suitable concentrators; however, some electrical power will probably be needed. Additional energy would be required for support operations, such as material acquisition, transport, and beneficiation, or conditioning of the oxygen for storage. To answer theenergy supply question, a more specific design and further analysis of possible tradeoffs are necessary.
While vapor phase pyrolysis is basically a continuous process, periodic shutdown is necessary for removal of the condensed suboxides from the cooling system.
In summary, vapor phase pyrolysis may be a viable process for producing oxygen from lunar materials. It is straightforward and does not require complex equipment. Its biggest disadvantage is the large amount of energy it requires. However, this energy requirement might be reduced by efficient use of solar energy, by recovering heat from the slag, and by using the recovered heat to preheat the feedstock. While such techniques would add complexity, the savings in energy might be worth the cost in added equipment. In any case, vapor phase pyrolysis clearly deserves additional development work.
![[NASA]](images/NASAball.gif)
![[Ames Research Center]](images/arclogo1.gif)
|
WebWork: Al Globus, Bryan Yager, and Tugrul Sezen |
![[LifeSciences]](images/lslogot.gif)
![[Space Settlement]](images/splogosm.gif)
|