Bioprocessing of Ores: Application to Space Resources
Karl R. Johansson
Introduction
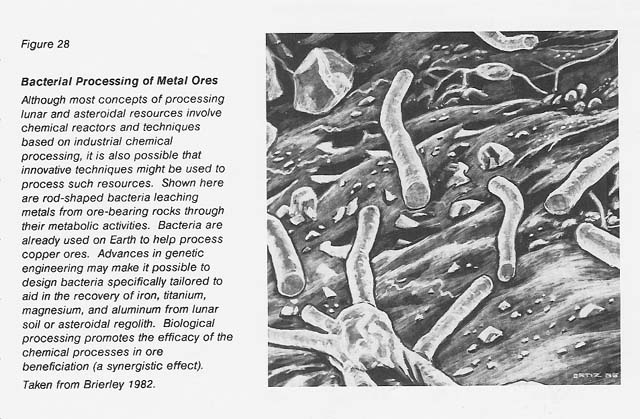
The role of microorganisms in the oxidation and leaching of various ores (especially those of copper, iron, and uranium) is well known. (Among the review articles and reference books on this subject are Brierley 1978 and 1982; Brock, Smith, and Madigan 1984; Decker 1986; Ehrlich 1981; Kelly, Norris, and Brierley 1979; Krumbein 1983; Lundgren and Silver 1980; and Torma and Banhegyi 1984.) This role is increasingly being applied by the mining, metallurgy, and sewage industries in the biobeneficiation of ores and in the bioconcentration of metal ions from natural receiving waters and from waste waters high in toxic metals (Belliveau et al. 1987, Ehrlich and Holmes 1986, Hutchins et al. 1986, Nicolaidis 1987, Olson and Brinckman 1986, Olson and Kelly 1986, Thompson 1986, Torma 1987a and b, Tsezos 1985, Volesky 1987, Woods and Rawlings 1985). See figure 28.

The question of harnessing bacteria for the beneficiation of ores on the Moon, on asteroids, or on Mars has been raised and must be seriously considered in the context of the utilization of space resources. Because of the almost total lack of organic matter on the Moon, it is fortunate that most bacteria known to participate in acid leaching of ores are autotrophic (derive all their carbon from carbon dioxide) as well as chemolithotrophic (derive energy through oxidation of reduced inorganic compounds or elements; e.g., hydrogen sulfide or ferrous ions). Furthermore, they satisfy all of their nutritional needs with inorganic substances, including certain trace elements known to be present in the Moon's regolith. But the development of biological processes to extract and purify ores on the Moon is severely constrained by the environmental conditions: the lack of elemental oxygen; the limited carbon, nitrogen, and hydrogen; the apparent lack of water; and the extremes of temperature and radiation. Thus, microbial ore processing must be established within a gas-tight enclosure. The enclosure must allow replenishment or augmentation of nutritional needs, retain moisture, maintain a suitable temperature, and protect the cells from radiation. See figure 29.

Various kinds of interactions between microorganisms and metals are known: (1) beneficial as well as toxic effects of metals on metabolism, (2) oxidation-reduction reactions, (3) solubilization of metals through acids produced by microbial growth, (4) precipitation of metals by pH increases, (5) conjugation of metals and organic compounds, (6) metabolic transformation of metals, and (7) accumulation of metals either on the inside or on the outside of cells. In this paper I will consider the processes that are particularly important to the technology of metal recovery. Some of them may have application in the space environment. However, essentially no research has been done with this application in mind.
Acid Leaching of Ores
Acid leaching is a hydrometallurgical process resulting. in the solubilization of ore minerals through chemical and biological oxidations and reductions of sulfur, iron, and certain other metals.
The Chemistry
The chemistry of the process is complex and greatly affected by pH! oxidation-reduction potential, dissolved oxygen, and temperature. (Some of the authorities describing this chemistry are Hutchins et al. 1986; Kelly, Norris, and Brierley 1979; Lundgren, Valkova- Valchanova, and Reed 1986; Torma and Banhegyi 1984; and Torma 1987a.) For example, bacteria can catalyze and drive the following oxidations:
|
|
|
The Bacteriology
The principal bacteria catalyzing reactions 1 through 5 are mixed populations of Thiobacillus ferrooxidans, Leptospirillum ferrooxidans, Thiobacillus thiooxidans, and several other species of acidophilic thiobacilli. There also. exist some thermophilic thiobacilli which are facultative autotrophs inasmuch as they can utilize certain organic substrates in the environment. Thiobacillus ferrooxidans is unique in that it derives energy from oxidations of sulfur and reduced iron, copper, and tin; it also fixes nitrogen. Thiobacillus thiooxidans cannot oxidize iron; rather, it oxidizes sulfur and probably zinc sulfide. Leptospirillum ferrooxidans will oxidize only the soluble form of iron (Fe + +), but in conjunction with certain other sulfur-oxidizing thiobacilli it will synergistically oxidize pyrite (FeS2) as well as chalcopyrite (CuFeS2).
Another group of bacteria, the genus Sulfolobus, which is widely distributed in volcanic vents and thermal springs, is able to oxidize sulfur and iron at temperatures of 80C, or even a few degrees higher. Sulfolobi are also able to grow in the absence of molecular oxygen provided M06 + or Fe + + + are present to serve as electron acceptors, thereby replacing O2 as the ultimate electron acceptor. They are facultative heterotrophs and occupy a unique niche in the bacterial kingdom as Archaebacteriaceae, a family possessing a cell membrane composed of a long-chain, ether- linked hydrocarbon monolayer (instead of a phospholipid bilayer) and lacking a peptidoglycan cell wall, which is found in all other bacteria (Kelly and Deming 1988). Whether these and certain other archaebacteria playa significant role in thermophilic leaching of ores can only be surmised.
A filamentous, moderately thermophilic, sulfur-oxidizing, autotrophic bacterium, Thermothrix thiopara, also flourishes in volcanic vents and in thermal springs (Brierley 1982) and may playa role in the leaching of ores. Similarly, the filamentous, mesophilic bacteria Thiothrix and Beggiotoa, which oxidize sulfide to elemental sulfur, have potential for ore beneficiation.
The Commercial Leaching Operation
The release and recovery of metals from ores are facilitated by methods designed to amplify the requisite oxidation-reduction reactions (Brierley 1978, Brierley 1982, Campbell et al. 1985).
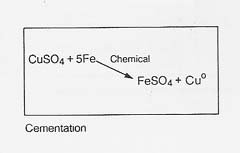
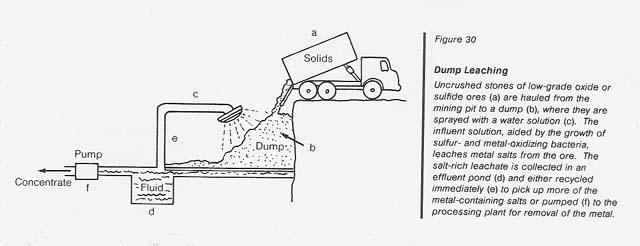
Dump leaching: This operation is usually applied to the extraction of copper from low-grade oxide or sulfide ores which are hauled as uncrushed stones from open-pit mines to enormous dumps (see fig. 30). The dumps are sprinkled with water and the percolate is collected in a natural or artificial catch basin. The copper is removed from the acidic leachate by cementation (with iron), electrolysis, or solvent extraction; the solution is then recycled through the dump. The operation continues for years during which time sulfur- and metal-oxidizing bacteria grow extensively to perpetuate the leaching process.
![]()
![[NASA]](images/NASAball.gif)
![[Ames Research Center]](images/arclogo1.gif)
|
WebWork: Al Globus, Bryan Yager, and Tugrul Sezen |
![[LifeSciences]](images/lslogot.gif)
![[Space Settlement]](images/splogosm.gif)
|