![[NASA]](images/NASAball.gif)
![[Ames Research Center]](images/arclogo1.gif)
|
WebWork: Al Globus, Bryan Yager, and Tugrul Sezen |
![[LifeSciences]](images/lslogot.gif)
![[Space Settlement]](images/splogosm.gif)
|
A Proposed Lunar Factory
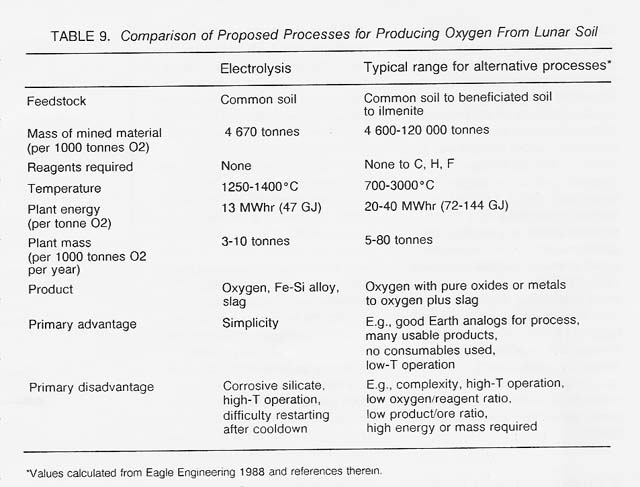
We envision a single-step, single- pot, steady-state electrolysis process using common lunar soil as feedstock with little or no preprocessing. As the soil is fed into the cell, it is melted by "excess" electrical heat released into the melt owing to resistance. The total electrode surface area would be about 30 square meters each (because each electrode is divided into fins, as in a car battery), and the total cell volume about 1 cubic meter. The operating temperature would be between 1300°C and 1600°C depending on the type of container and electrode materials that are ultimately developed. The cell would produce 1.4 tonnes iron-silicon metal, 1 tonne oxygen, and about 3.5 tonnes slag in 24 hours, with an energy requirement of about 13 MWhr (or 47 GJ). The process would satisfy many of the criteria set forth above for early lunar technologies, including use of common and easily mined lunar soil as feedstock, absence of a need to supply reagents from Earth, and simplicity of the process combined with multiple products. Mass, size, and power requirements of the process are also competitive with alternative processes -(table 9). The low energy and mass requirements of the process are particularly important because the major expense in establishing a lunar oxygen factory is the cost of transporting the plant materials (including the required power plant) to the Moon (see Simon's discussion in volume 2).
Conclusions
All the processes that have been suggested for extracting oxygen from lunar materials and probably many that haven't yet been suggested deserve our careful consideration in determining which is the "best" process to be implemented on the Moon. However, all the processes require substantial additional study before we are able to judge their relative worth for extracting lunar oxygen; and, before an operational plant can be built, even more study will be required.
We note that there is not much time (we hope) before the chosen process will be needed on the Moon. If we are to ensure that an oxygen production plant is included in the early planning and development of a lunar base, we need to progress quickly in assessing the various proposed processes so that the concept of a lunar oxygen plant can become a part of everyone's idea of what a lunar base should be.
Although it is certainly too early to decide which oxygen extraction process is the best one, our preliminary work with magma electrolysis has increased our confidence in its promise. We feel that its theoretical advantages listed above, including relatively low energy requirements, low mass, simplicity, and versatility with respect to feedstock, are sufficient to warrant its consideration as one of the processes most likely to be used in the early mining of oxygen from the Moon.
References
Arnold, James. 1980. The Frontier in Space. Am. Sci. 68:299-304.
Blacic, James D. 1985. Mechanical Properties of Lunar Materials Under Anhydrous, Hard Vacuum Conditions: Applications of Lunar Glass Structural Components. In Lunar Bases and Space Activities of the 21st Century, ed. W. W. Mendell, 487-495. Houston: Lunar & Planetary Inst.
Bova, Ben. 1987. Welcome to Moonbase. New York: Ballantine.
Burt, D. M. 1990. Lunar Mining of Oxygen Using Fluorine. In The 2nd Conference on Lunar Bases and Space Activities of the 21st Century, ed. W. W. Mendell. Houston: Lunar & Planetary Inst. (in press).
Burt, Donald M. 1989. Mining the Moon. Am. Sci. 77:574-579.
Colson, Russelll O, and Larry A. Haskin. 1990. Lunar Oxygen and Metal for Use in Near-Earth Space: Magma Electrolysis. fn Engineering, Construction, and Operations in Space, Proc. Space 90, ed. Stewart W. Johnson and John P. Wetzel. New York: American Soc. Civil Eng. (in press).
Criswell, David R., and Robert D. Waldron. 1985. Concept of the Lunar Power System. Space Solar Power Review 5:53-75.
Cutler, Andrew Hall, and Peter Krag. 1985. A Carbothermal Scheme fQIILunar Oxygen Production. In Lunar Bases and Space Activities of the 21st Century, ed. W. W. Mendell, 559-569. Houston: Lunar & planetary tnst.
Davis, Hubert P. 1983. Lunar Oxygen Impact Upon STS Effectiveness. Report EEl 83-63. Houston: Eagle 'Engineering, Inc., May.
Eagle Engineering, .Inc. 1988. Conceptual Design of a Lunar Pilot Plant, Lunar Base Systems Study (LBSS), Task 4.2. EEl 88-182. Houston.
French, Bevan M. 1977. The Moon Book. Westford, MA: Murray Printing Co.
Gibson, Michael A., and Christian W. Knudsen. 1990. Lunar Oxygen Production from Ilmenite. In The 2nd Conference on Lunar Bases and Space Activities of the 21st Century, ed. W. W. Mendell. Houston: Lunar & Planetary Inst. (in press).
Haskin, Larry A. 1985. Toward a Spartan Scenario for Use of Lunar Materials. In Lunar Bases and Space Activities of the 21 st Century, ed. W. W. Mendell, 435- 443. Houston: Lunar & Planetary Inst..
Haskin, Larry A. 1990. Water and Cheese From the Lunar Desert: Abundances and Accessibility of H, N, and C on the Moon. In The 2nd Conference on Lunar Bases and Space Activities of the 21st Century, ed. W. W. Mendell. Houston: Lunar & Planetary Inst. (in press).
Haskin, Larry A., and Russell O. Colson. 1990. Lunar Resources- Toward Living Off the Lunar Land. Proc. 1st Symp. NASA/Univ. of Arizona Space Engineering Research Center, ed. Terry Triffet. Tucson (in press).
Haskin, Larry A.; Russell O. Colson; David J. Lindstrom; Robert H. Lewis; and Krystyna W. Semkow. 1990. Electrolytic Smelting of Lunar Rock for Oxygen, Iron, and Silicon. In The 2nd Conference on Lunar Bases and Space Activities of the 21st Century, ed. W. W. Mendell. Houston: Lunar & Planetary Inst. (in press).
Harz, Friedrich. 1985. Lava Tubes: Potential Shelters for Habitats. In Lunar Bases and Space Activities of the 21st Century, ed. W. W. Mendell, 405-412. Houston: Lunar & Planetary Inst.
Johnson, Francis S. 1980. Science and Development. In Space and Development, ed. Yash Pal, 3-10. New York: Pergamon.
Keller, Rudolf. 1989. Dry Extraction of Silicon and Aluminum From Lunar Ores. EMEC Consultants Final Report to Johnson Space Center on NASA contract NAS9-17811.
Lin, T. D. 1985. Concrete for Lunar Base Construction. In Lunar Bases and Space Activities of the 21st Century, ed. W. W. Mendell, 381-390. Houston: Lunar & Planetary Inst.
Mendell, W. W., ed. 1985. Lunar Bases and Space Activities of the 21st Century. Houston: Lunar & Planetarv Inst.
Nozette, So, ed. 1983. Defense Applications of Near-Earth Resources, the report of a workshop held at the California Space Institute Aug. 15-17, 1983, sponsored by the Institute for Defense Analyses. CSI83-3.
Steurer, Wolfgang H. 1985. Lunar Oxygen Production by Vapor Phase Pyrolysis. In Space Manufacturing 5: Engineering with Lunar and Asteroidal Materials, Proc. 7th Princeton/AIM/SSI [Space Studies Institute] Conf., ed. Barbara Faughnan and Gregg Maryniak, 123-131. New York: AIAA.
Thompson, G. V. E. 1951. The Lunar Base. J. Brit. Interplanet. Soc. 10:49-70.
Waldron, Robert D. 1985. Total Separation and Refinement of Lunar Soils by the HF Acid Leach Process. In Space Manufacturing 5: Engineering with Lunar and Asteroidal Materials, Proc. 7th Princeton/AIAA/SSI [Space Studies Institute] Conf., ed. Barbara Faughnan and Gregg Maryniak, 132-149. New York: AIM.
Wittenberg, L. J.; J. F. Santarius; and G. L. Kulcinski. 1986. Lunar Source of 3He for Commercial Fusion Power. Fusion Technology 10:167-178.
![[NASA]](images/NASAball.gif)
![[Ames Research Center]](images/arclogo1.gif)
|
WebWork: Al Globus, Bryan Yager, and Tugrul Sezen |
![[LifeSciences]](images/lslogot.gif)
![[Space Settlement]](images/splogosm.gif)
|