Temperature
Some catalyst activity was noted as low as 200°C; the catalyst
was found to be very active at 250°C; so excellent conversions were obtained.
Therefore, all the runs were made at a nominal catalyst bed temperature of 250°C,
except run 57, which was made at 350°C. In run 57 we tried to increase the conversion
at a 3: 1 hydrogen- carbon monoxide mole ratio and a 1500-hr^-1
space velocity by increasing the temperature; however, the conversion of carbon
dioxide to methane and water decreased as the temperature was increased.
Pressure
The first nine runs were made at atmospheric pressure. The
conversions were nearly complete at a 4: 1 mole ratio even with space velocities
of 2000 hr^-1. It was only at lower hydrogen-carbon monoxide mole
ratios that the conversions decreased sufficiently to require raising the catalyst
bed pressure. The last three runs were made at 6.1 atm to approach complete
conversion at a 3: 1 ratio. In comparing runs 54 and 55, it can be seen that
increasing the pressure from 1 to 6 atm decreased the carbon dioxide yield from
0.8 to 0.4 percent and correspondingly increased the yields of water and methane.

Hydrogen-Carbon Monoxide Mole Ratio
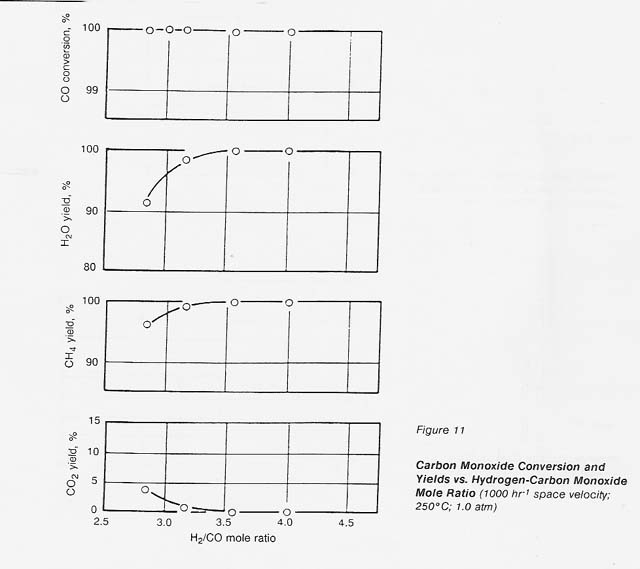
The effect of hydrogen-carbon monoxide mole ratio on conversion and yields can be seen in figure 11. At a space velocity of 1000 hr-1, at 250°C and 1.0 atm, the catalyst gave complete conversion of carbon monoxide and carbon dioxide until we decreased the hydrogen-carbon monoxide mole ratio to less than 3.5: 1. The carbon monoxide conversion remained complete but the carbon dioxide yield increased; at a 3: 1 ratio, the carbon dioxide yield was approximately 2 percent.
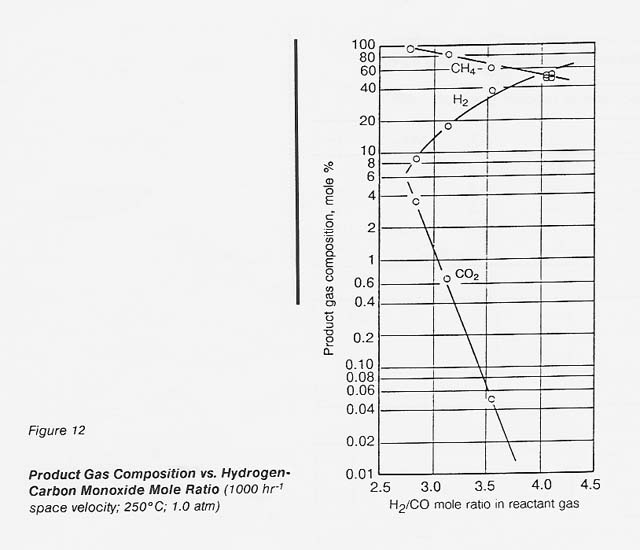
The effect of hydrogen-carbon monoxide mole ratio on the product gas composition can be seen in figure 12. No carbon monoxide could be detected in the outlet gas for any of these runs. Within this range, the carbon dioxide content of the gas increased logarithmically as the hydrogen-carbon monoxide mole ratio was decreased below 3.5:1 (to about 1.5% at 3:1). The theoretical product yield at a 3: 1 ratio is 100 percent methane, a percent hydrogen. The catalyst gave 86 percent methane, 13 percent hydrogen at the 3: 1 ratio.
Space Velocity*
At a 4: 1 mole ratio, no carbon dioxide was formed at space velocities up to 2000 hr-1. At a 3:1 ratio, the carbon dioxide yield increased rapidly as the space velocity was increased above 1000 hr^-1.
Material Balance
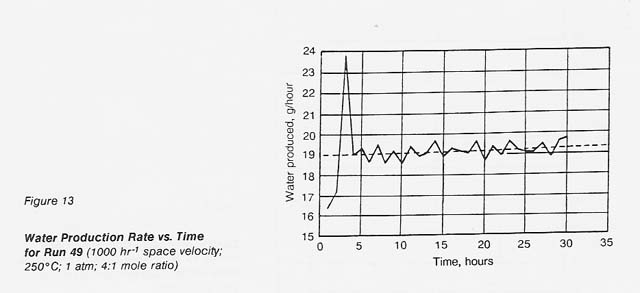
With the exception of two runs, all overall material balances for the runs (see table 1) were under 100 percent. Most of the low material balances can be attributed to low water recoveries. Because the catalyst is known to be a good adsorbent for water, we hypothesized that some of the water was slowly being adsorbed on the catalyst. In order to prove that this was the case, a long- duration run (run 49) was made. See figure 13. The water production, which fluctuated about +-0.5 g/hr, gradually increased throughout the run (dotted line). After 30 hours, the liquid water production rate was 19.2 g/hr (about 96% of theoretical). At the rate of increase of water production (0.01 g/hr), it would have taken about 100 hr before the actual water production rate equaled the theoretical production rate. For long runs, the water balance should be no problem; in fact, we hypothesize that the small amount of water adsorbed on the catalyst may he(p to prevent carbon formation.
Heat Balance
In all runs, the majority of the heat was released in the initial third of the bed; however, in several runs at high space velocity (1500 or 2000 hr-1) or low hydrogen-carbon monoxide mole ratios (3: 1) or both, enough heat was released in the middle third of the catalyst bed to require some cooling. At the highest space velocities, temperature control was very difficult, because of the large amount of cooling air required to maintain the nominal catalyst bed temperature.
Pressure Drop
The relatively low pressure drop across the catalyst bed was excellent. It did not go up with time even at hydrogen-carbon monoxide mole ratios as low as 3: 1. Run 49 was continued for 31 hours without shutdown; the pressure drop did not increase a measurable amount during this long period. The absence of a pressure buildup at the catalyst bed indicated no carbon deposition and a long, useful catalyst life.
*Space velocity is a measure of reactor capacity. It is the reciprocal of space time, which is defined as the time elapsed in processing one reactor volume of feed at specified conditions. Thus, space velocity is the number of reactor volumes of feed that can be processed within a given time. The higher the space velocity the better, provided the desired reaction occurs.
![[NASA]](images/NASAball.gif)
![[Ames Research Center]](images/arclogo1.gif)
|
WebWork: Al Globus, Bryan Yager, and Tugrul Sezen |
![[LifeSciences]](images/lslogot.gif)
![[Space Settlement]](images/splogosm.gif)
|